Tokyo, June 1, 2024 - Medicaroid, a Japanese surgical robot company, announced today that its Hinotori surgical robot has been approved for insurance coverage for use in thoracic surgeries in Japan.
This follows the approval by Japan's Ministry of Health, Labour and Welfare (MHLW) in April 2024 to expand the indications for which the surgical robot can be used, including thoracic surgical procedures.
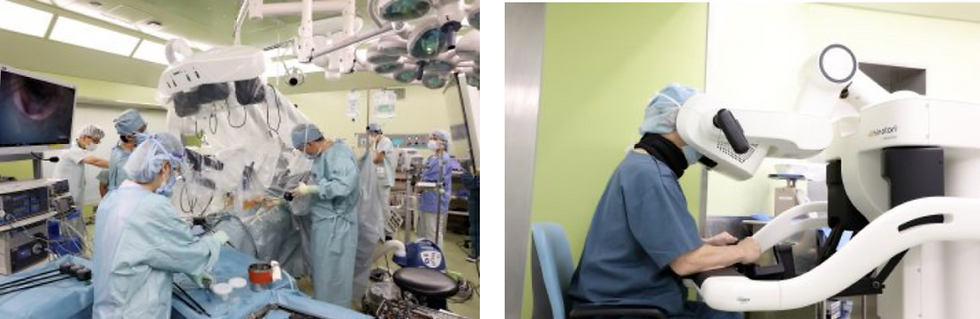
Hinotori Surgical Robot
Developed by Medicaroid, the Hinotori surgical robot is Japan's first domestically produced surgical assistant robot system. The system comprises three main components:
Operating Unit: Designed with compact mechanical arms that do not occupy much space, these arms alleviate the issues of interference commonly seen with longer arms in traditional systems like the Da Vinci robots.
Control Console: Ergonomically designed to adjust seamlessly to the surgeon's posture, creating a stress-free surgical environment.
Visual and Control Unit: Features a 3D high-definition imaging system that enhances communication between the surgeon, assistants, and other medical staff during operations.
Key Features of the Hinotori Surgical Robot include:
Open Operating Space: Unique compact arm configuration provides a wide operating space, enhancing efficiency.
Reduced Arm Interference: The eight-degree-of-freedom arm moves smoothly, minimizing interference with other arms and assistants.
Dock-Free Design: The pivotal points are software-controlled, ensuring clear workspace around the sleeve needles without the need for physical docking.
High-Definition Imaging: The full HD 3D system offers precise visuals to aid in surgeries.
Ergonomic Design: The Surgeon Cockpit is optimized for human physical characteristics.
The robot is also equipped with the SOT-100 Vercia surgical table, designed specifically for surgery with various modes to assist in patient positioning during different surgical settings, including hybrid operating rooms.
Medicaroid hopes that the Hinotori will replace the Da Vinci surgical robots in the Japanese market and meet the specific needs of Japanese surgeons.
Recent Developments
On September 22, 2023, Medicaroid announced that its Hinotori surgical robot system had received regulatory approval from the Health Sciences Authority (HSA) of Singapore for use in urology, gastroenterology, and gynecology surgeries.
On October 11, remote surgical demonstrations were conducted using the Hinotori surgical robot system in Singapore and Japan.
These demonstrations utilized a surgical console installed at the National University of Singapore and a Medicaroid smart lab at Fujita Health University in Japan. A novice surgeon in robot-assisted surgery performed the procedure under the real-time guidance of Professor Ichiro Uyama at the Hisen-Mikuni Remote Robotics Experimental Facility in Tokyo.
The Hinotori surgical robot was first approved in Japan in August 2020 for use in urological surgeries and later expanded to gastroenterology and gynecology in October 2022.
About Medicaroid
Medicaroid originated as the "Medical Robotics Research Group" in 2012, a collaboration between Yasuhiko Hashimoto, the current President/CEO of Kawasaki Heavy Industries, and Kaoru Asano, the current Senior Executive CTO of Sysmex Corporation. Established in 2013 as a joint venture between Kawasaki Heavy Industries, a leader in industrial robotics, and Sysmex Corporation, known for its extensive network in medical testing and diagnostics, Medicaroid aims to innovate in the field of robotic-assisted surgery.










Comments